- 17min
- 11777
- 0
Rechargeable Batteries or non-Rechargeable Batteries , are so common these days that we hardly notice them. They are, nevertheless, a magnificent innovation with a lengthy and illustrious past and an equally enthralling future.
Batteries are one of the most important vital organs for Cordless instruments such as rechargable drill.
A battery, in general, is a device that stores chemical energy before converting it to electricity Rechargeable Batteries are essentially miniature chemical reactors that produce energetic electrons that can flow via an external device.
Batteries Through History
Luigi Galvani, an Italian physician who inadvertently found that muscles contract when two different metals are contacted, laid the framework for the construction of modern batteries in 1780. Galvani accidently created the “galvanic cell” with his “frog leg experiment.”
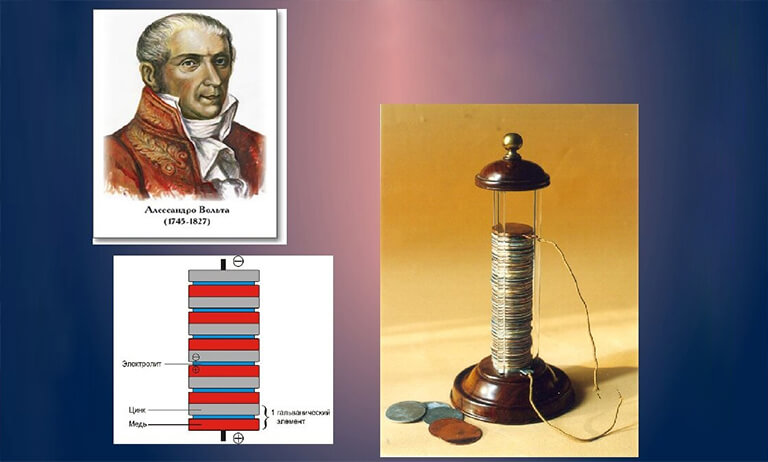
A circuit was created by combining two separate electrodes (the two metals) with an electrolyte (the water in the frog’s legs). Allesandro Volta created the Volta Column in 1800 based on this understanding. In a sandwich configuration, many “galvanic cells” were layered on top of one another. Each cell was made up of a copper and zinc sheet as well as a salt-soaked cardboard disc. A constantly flowing electric circuit was formed by connecting the highest and lowermost cells with a wire. The higher the electrical voltage, the more cells the column had — the forerunner of today’s batteries was born.
What is the mechanism of a battery?
A typical battery is made up of two metals or compounds with distinct chemical potentials that are separated by a porous insulator. When electrons are permitted to travel via the linked external device, the chemical potential is the energy contained in the atoms and bonds of the compound, which is subsequently given to the moving electrons.
The electrolyte is a conducting fluid like as salt or water that is used to move soluble ions from one metal to the other during the reaction.
The anode is the metal or compound that loses electrons during discharge, whereas the cathode is the metal or compound that absorbs electrons. Our electronic gadgets are powered by the passage of electrons from the anode to the cathode via the external connection.
Technical Terms Used When Working with Batteries
There are certain distinct terminologies that identify the features of a battery, such as Ampere-hour (mAh), C-rating, voltage, charging voltage, charging current, discharging current, cut off voltage, shelf life, and cycle life, to name a few.
✅ Voltage: The term “battery voltage” refers to the difference in electrical potential between the positive and negative terminals of a battery. A large potential difference results in a greater voltage. Electric potential is the difference in charge between two points – in this case, the two poles of a battery. One is positively charged, the other is negatively charged. A negative charge simply means that there is an excess of negatively charged particles, or electrons, on the terminal, while a positively charged terminal is devoid of these electrons.

✅ Power Capacity: The battery charge capacity is measured in amp hours. We can theoretically draw 5 amps constantly for 60 minutes before draining our 5amp hour battery. That’s under ideal circumstances: optimal temperature, no vibration, and constant power. Because you never reach those perfect circumstances in the actual world, we call it a competent charge. Consider amp hour as a measure to determine whether one battery can keep a charge longer than another. We may get a battery’s amp hour rating by multiplying the current by the discharge time, or we can determine how long the battery will survive while producing a specific level of current.
✅ Shelf Life: There may be instances where batteries are left idle/sealed for extended periods of time, particularly at stores/shops. So, shelf life refers to the amount of time a battery may be charged and used for a specified period of time. Non- Rechargeable Batteries have a longer shelf life since they are used and thrown. Even if the shelf life of Rechargeable Batteries is reduced, we can still recharge them.
✅ Cycle Life: If a battery is completely charged and subsequently drained to 80% of its original capacity, the battery is considered to have completed one cycle. Similarly, the cycle life of a battery is defined by the number of such cycles that it can charge and discharge. The battery’s quality will improve as the cycle life increases. However, if a battery is depleted to 40% of its true capacity after being completely charged at the start, it cannot be termed a cycle life.

Different Types of Batteries
Batteries are divided into two categories: rechargeable batteries and non-rechargeable batteries .
- Non-rechargeable batteries/ Batteries that can’t be recharged (primary batteries)
- Rechargeable batteries / Batteries that can be recharged (secondary batteries)
Non-rechargeable Batteries
Because they can only be used once, these sorts of batteries are referred to as primary batteries.
Alkaline Rechargeable Batteries: It is primarily made up of Zinc (Zn) and Manganese dioxide (MnO2), and the electrolyte used is potassium hydroxide, which is a fully alkaline material. As a result, the battery is known as an alkaline battery with a power density of 100 Wh/Kg. It may be found in flashlights, remote controls, wall clocks, and other tiny portable devices.
Coin cell batteries: Coil cell batteries have an alkaline chemical composition as well. Apart from alkaline materials, lithium and silver oxide chemicals will be employed in the production of these batteries, which will be more efficient in supplying a constant and stable voltage in such a small size. It has a 270 Wh/Kg power density. Watches, wall clocks, weighing machines, and other low-power small electronic items, among other things, use these batteries.

Rechargeable Batteries
These rechargeable batteries are referred to as secondary Rechargeable Batteries since they may be recharged and reused. Though they are expensive, they can be recharged and reused, and when properly used and charged, they have a long-life expectancy.
Lead-Acid Batteries : It is made out of lead-acid, which is a fairly inexpensive material that is used to power the lighting systems in cars and trucks. These are preferred in items when size, space, and weight are unimportant. The nominal voltage ranges from 2V to 24V, and the most popular types are 2V, 6V, 12V, and 24V batteries. It has a 7 Wh/Kg power density.
Common uses: Cars, UPS (uninterrupted Power Supply), robots, heavy machinery, and other applications.
Benefits:
- Cost-effective
- Rechargeable with ease
- High-output power capabilities
Drawbacks:
- very heavy
- occupies a lot of space
- has a low power density
- Nickel-Cadmium (NiCAD) Batteries: In 1899, a Swedish scientist invented the world’s first NiCad battery. Needless to say, a great deal has changed since then. A nickel oxide-hydroxide positive electrode plate, a cadmium negative electrode plate, a separator, and a potassium hydroxide electrolyte make up a conventional nickel-cadmium battery.
📌 Common uses: Toys, emergency lights, medical equipment, commercial and industrial items, electric razors, two-way radios, power tools, and more devices use NiCAD batteries.
Benefits: Some of the benefits of NiCAD batteries are summarized below:
- High number of charge/discharge cycles: if correctly maintained, the NiCd battery may give over 1000 charge/discharge cycles.
- Exceptional load performance: NiCd enables for recharging at extremely low temperatures.
- Long shelf life: at any charge level.
- Simple storage and transport: most airfreight carriers accept NiCd without any particular requirements.
- Exceptional low-temperature performance; forgiving if abused: the NiCd is one of the most durable rechargeable batteries available.
- In terms of cost per cycle, the NiCd battery is the most cost-effective option.
- Most NiCd cells are cylindrical and come in a variety of sizes and performance choices.
Drawbacks: And here are a few of their flaws:
Low energy density as compared to modern systems.
Remembering effect: the NiCd must be trained on a regular basis to avoid memory.
Harmful to the environment: NiCd includes hazardous elements.
The use of NiCd batteries is restricted in several countries.
Has a high self-discharge rate; requires recharging after storage

- Nickel-Metal Hydride (NiMH) Batteries: NiMH batteries are a relatively newer invention. The Battelle-Geneva Research Center began research and development in 1967 and finished it properly in 1987. A nickel hydroxide positive electrode plate, a hydrogen ion negative electrode plate, a separator, and an alkaline electrolyte such as potassium hydroxide make up the chemical composition of a conventional nickel-metal hydride battery.
✔ Common uses: Automobile batteries, medical equipment, pagers, cell phones, camcorders, digital cameras, electric toothbrushes, and other low-cost consumer products are among them.
Benefits: Here are just a handful of the benefits of using a NiMH battery:
- Approximately 30–40% more capacity than a conventional NiCd. NiMH offers the potential to achieve much greater energy densities.
- Unlike NiCd, it is less prone to memory. Periodic workout cycles are no longer necessary.
- Simple storage and transportation: the circumstances of transit are not regulated.
- Environmentally friendly: only minimal toxins are present; recycling is lucrative
Drawbacks: Here are some of the drawbacks:
- Limited service life: after 200 to 300 cycles of deep cycling, especially at high load currents, the performance begins to decrease. Deep discharge cycles are favored over shallow discharge cycles.
- Low discharge current: while a NiMH battery may generate high discharge currents, frequent discharges with high load currents shorten the battery’s cycle life. Load currents of 0.2C to 0.5C produce the best performance (one-fifth to one-half of the rated capacity).
- A more sophisticated charge algorithm is required since the NiMH produces more heat during charging and takes longer to charge than the NiCd. The trickle charge is extremely important and must be properly managed.
- High self-discharge: When compared to NiCd, the NiMH has a 50 percent greater self-discharge. New chemical additions increase self-discharge but reduce energy density.
- If stored at high temperatures, performance degrades: NiMH batteries should be kept cold and charged to around 40% capacity.
- High maintenance: To avoid crystalline formation, the battery must be fully discharged on a regular basis.
- About 20% more costly than NiCd: NiMH batteries intended for high current consumption cost about 20% more than conventional NiCd batteries.
💠💠💠 v Lithium-ion Battery: A lithium-ion battery, often known as a Li-ion battery, is a rechargeable battery in which lithium ions travel from the negative electrode to the positive electrode via an electrolyte during discharge and then back again during charging. The positive electrode of a lithium-ion battery is made of an intercalated lithium compound, whereas the negative electrode is usually made of graphite.

Lithium and Lithium-ion Batteries, What’s the Difference?
The most significant distinction between Lithium batteries and Lithium-ion batteries is that Lithium batteries are single-use and cannot be recharged after they have been used. Lithium-ion batteries, on the other hand, may be used again because they are rechargeable rechargeable and can be charged and discharged hundreds of times.
Lithium batteries live significantly longer while they’re resting on a shelf unused. Lithium batteries may last 10 to 12 years while still holding a charge, however lithium-ion batteries only last 2 to 3 years. You might not realize that these two types of Lithium batteries aren’t the only ones available; learn more about the differences between Lithium-ion and Lithium-polymer batteries in our post.
Despite the fact that Lithium-ion batteries appear to be much superior, especially in terms of waste, there are still several qualities that make Lithium batteries quite useful. They have a far greater energy density than lithium-ion batteries, which means they can retain a lot more charge for their size. Even if it’s the only charge they’ll ever have, lithium batteries can deliver power from a single charge. Unfortunately, there is no safe or practical way to recharge Lithium-ion batteries, which is why they were created in the first place. Lithium batteries are also less expensive to buy since they are cheaper and easier to produce.
Lithium Ion Battery Advantages and Disadvantages
Benefits: Here are just a handful of the benefits of using a Lithium Ion Battery
- It has a high energy density, two times that of Ni-Cd.
- The Lithium-Ion battery may be charged several times.
- There isn’t any sort of memory impact. As a result, there is no need to fully drain them before recharging.
- The battery can withstand several charge/discharge cycles; • The battery can maintain a charge and lose just 5% of its charge every month.
Drawbacks: Here are some of the drawbacks:
It only lasts two to three years after being manufactured, and it is temperature sensitive.
Once a battery has been entirely drained, it cannot be recharged.
It is reasonably priced.
If the “separator” is damaged, it might catch fire.
Li-ion batteries Application
Li-ion batteries are lightweight power sources with a high energy density that may be used in a range of applications. Connecting multiple tiny batteries in a parallel circuit to power bigger equipment, such as electric automobiles, is more effective and efficient than connecting a single huge battery.
- Power Tools: Cordless drills, sanders, saws, and a range of garden tools, such as whipper-snippers and hedge trimmers, all require Li-ion batteries.
- Portable Devices: Mobile phones and smartphones, computers and tablets, digital cameras and camcorders, e-cigarettes, portable game consoles, and torches are just a few of them (flashlights).
- Electric Vehicles: In electric automobiles, hybrid vehicles, electric motorcycles and scooters, electric bicycles, personal transporters, and sophisticated electric wheelchairs, batteries are employed. Radio-controlled models, model airplanes, planes, and the Mars Curiosity rover are also on display.

Cordless Power Tool Battery
The battery is what allows cordless tools to function, selecting the sort of battery technology that the tool employs is almost as crucial as selecting the tool itself. When it comes to choosing a cordless power tool you want to be sure you get the right one for the desired application. One of the most significant features and specifications to think about is the type of the battery you’ll be using. Nickel-Cadium (NiCD), Nickel-Metal Hydride (NiMH), and Lithium-ion are the three major battery types used in cordless power tools (Li-ion).
📌 Long battery life is only one of the numerous features that compliment cordless power tools, which are becoming increasingly popular. As a result, another shift in the power tool industry is underway, with lithium-ion batteries replacing nickel-cadmium battery is smaller and lighter than its predecessor, and it doesn’t self-discharge, thus it can be kept for months without losing its charge. Lithium ion batteries have a higher energy density, or Watt hours per unit of volume, than other types of batteries, making them more efficient.
This allows the battery to draw greater current and function for longer periods of time than a Nickel-Cd battery. The typical Lithium ion battery lasts for 12 months, but the NiCd battery only lasts a few months. There are hundreds of lithium ion batteries on the market, all with distinct voltages and capacities. Keep in mind that other features that affect a battery’s total life and run time are generally the deciding considerations when power tool battery purchasers make selections (apart from price, of course), therefore most of the qualities that are listed here, have some bearing on how long a battery will last.
Operating Cordless Power Tools; Use Original Manufacturer Batteries
When a third-party battery is charged with a manufacturer’s charger, it might overheat, shortening its life or perhaps causing it to fail completely. In Addition, if the risk of failure or lower performance and life aren’t enough, using knock-off batteries has further drawbacks. For starters, the battery, tool, and charger are no longer authorized by the UL or CSA. Each tool is put through rigorous testing, which includes a battery test from the original manufacturer. Third-party batteries aren’t usually held to the same standards, which can lead to fires, property damage, and human harm. When you use a knock-off battery on a tool or charger, you risk voiding the warranty on the tool or charger.
FAQ ❓
What’s the difference between Amp Hour and Amperage?
The greatest level of energy that a battery is designed to provide at a given instant in time for a short length of time is indicated by its amperage rating. Meanwhile, the Ampere Hour rating indicates how many amps a battery can produce over a period of time.
How to figure out how much lithium is in a battery?
A battery or battery pack’s lithium (or lithium equivalent) content may be calculated as 0.3 x amp hour capacity.
Is it true that keeping batteries in the fridge extends their life?
The self-discharge rate of a zinc-carbon (including zinc-chloride) or lithium battery can be lowered by keeping it cold. Remember, don’t use a battery directly from the refrigerator; instead, let it to slowly warm up to room temperature (a few hours are needed for a unit to warm right through).
Why do lead acid batteries not live indefinitely?
Over time, all rechargeable batteries deteriorate. There are no exceptions when it comes to lead acid and sealed lead acid batteries. Keep in mind that, the fact that a lead acid battery can no longer power a certain item does not imply that the battery is depleted of energy. If you short the terminals on a vehicle battery that won’t start the motor, you may still get a lot of fireworks.
Is it true that all batteries are recyclable?
No. The types of metals utilized in the battery construction have a big impact. Lead is highly recyclable, with old batteries accounting for around half of the lead utilized in new ones. Nickel Cadmium, on the other hand, is too costly to remove and reuse.
Conclusion 🧾
Rechargeable batteries serve a variety of functions in modern day daily life, ranging from delivering the initial power required to start automobile engines to serving as a backup source of electricity in telecommunications, public transit, and medical treatments. The development of a fundamentally more sustainable battery that addresses challenges like supply chain volatility and material abundance while outperforming Li-ion batteries is a priority. Next-generation batteries that don’t rely on strategic components like Li might help the industry transition to more sustainable models. Future battery technology will have to overcome severe scientific hurdles.

Ronix
6 December 2021









Thanks for sharing Relevant Information
Very helpful